Identify a balanced equation for the calcination of NiCO3.
A) NiCO3(s) + CaO(s) 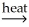 NiO(s) + CaCO3(s)
NiO(s) + CaCO3(s)
B) NiCO3(s) 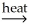 NiO(s) + CO2(g)
NiO(s) + CO2(g)
C) NiCO3(s) + C(s) 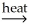 NiO(s) + 2CO(g)
NiO(s) + 2CO(g)
D) NiCO3(s) + SiO2(s) 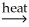 NiSiO3(s) + CO2(g)
NiSiO3(s) + CO2(g)
E) NiCO3(s) + CO(g) 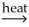 NiO(s) + 2CO2(g)
NiO(s) + 2CO2(g)
Correct Answer:
Verified
Q24: Why can't chromium and nickel form a
Q28: Which of the following is TRUE concerning
Q31: What are the components of bronze?
A) copper
Q32: Which of the following is TRUE concerning
Q34: Identify the normal crystal structure at room
Q39: Identify a balanced reaction for the smelting
Q44: What is the difference between ferromagnetism and
Q50: Why does a "two-phase" structure occur in
Q57: Why is zinc used to coat steel
Q59: What is an advantage of hydrometallurgical processes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents