A substance has a melting point of 20°C and a heat of fusion of 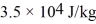 .The boiling point is 150°C and the heat of vaporization is
.The boiling point is 150°C and the heat of vaporization is 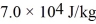 at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are
at a pressure of 1.0 atm.The specific heats for the solid,liquid,and gaseous phases are 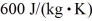 ,
, 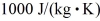 ,and
,and 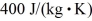 ,respectively.The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C,at a pressure of 1.0 atmosphere,is closest to
,respectively.The quantity of heat given up by 0.50 kg of the substance when it is cooled from 170°C to 88°C,at a pressure of 1.0 atmosphere,is closest to
A) 70 kJ.
B) 14 kJ.
C) 21 kJ.
D) 30 kJ.
E) 44 kJ.
Correct Answer:
Verified
Q33: Two experimental runs are performed to determine
Q34: A substance has a melting point of
Q35: A certain metal has a coefficient of
Q36: A 400-g piece of metal at 120.0°C
Q37: A person makes ice tea by adding
Q39: An 80-g aluminum calorimeter contains 380 g
Q40: Two experimental runs are performed to determine
Q41: How many grams of ice at -13°C
Q42: A spherical object 25.0 cm in diameter
Q43: A heat conducting rod,0.90 m long,is made
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents