A person pours 330 g of water at 45°C into an 855-g aluminum container with an initial temperature of 10°C.The specific heat of aluminum is 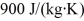 and that of water is
and that of water is 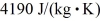 .What is the final temperature of the system,assuming no heat is exchanged with the surroundings?
.What is the final temperature of the system,assuming no heat is exchanged with the surroundings?
A) 28°C
B) 32°C
C) 31°C
D) 33°C
E) 35°C
Correct Answer:
Verified
Q17: An architect is interested in estimating the
Q18: A brass rod is 40.1 cm long
Q19: A chunk of ice (T = -20°C)is
Q20: The coefficient of linear expansion of aluminum
Q21: Heat is added to a 2.0 kg
Q23: If we use 67 W of power
Q24: If you add 700 kJ of heat
Q25: Heat is added to a pure substance
Q26: A 905-g meteor impacts the earth at
Q27: If 2.0 g of water at 0.00°C
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents