Multiple Choice
A sealed 89- 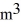 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 350 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is
tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K.The gas is heated to a final temperature of 350 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is 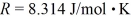 =
= 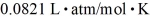 .The initial pressure of the gas is closest to
.The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
Correct Answer:
Verified
Related Questions
Q5: A mole of oxygen (O2)molecules and a
Q6: If we double the root-mean-square speed (thermal
Q7: If the temperature of a fixed amount
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents