An ideal gas is at a pressure 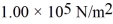 and occupies a volume 2.00 m3.If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
and occupies a volume 2.00 m3.If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
A) 0.500 × 105 N/m2
B) 4.00 × 105 N/m2
C) 1.00 × 105 N/m2
D) 2.00 × 105 N/m2
E) The answer depends on the mass of the gas particles.
Correct Answer:
Verified
Q28: Sometimes an experiment requires a certain pure
Q29: The root-mean-square speed (thermal speed)of a certain
Q30: A 0.10- Q31: What is the average translational kinetic energy Q32: 3.00 moles of an ideal gas at Q34: A sealed container holds 0.020 moles of Q35: How many moles of water (H2O)molecules are Q36: A hot air balloon has a volume Q37: The figure shows a 50-kg frictionless cylindrical Q38: What is the mass density of argon![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents