Sometimes an experiment requires a certain pure gas to be used at reduced pressure.One way to achieve this is to purchase a sealed glass container filled with the gas,and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet.If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 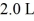 ,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
A) 33 kPa
B) 50 kPa
C) 300 kPa
D) 200 kPa
Correct Answer:
Verified
Q23: An ideal gas is kept in a
Q24: A 25-L container holds ideal hydrogen (H2)gas
Q25: What is the total translational kinetic energy
Q26: A 5.0-liter gas tank holds 1.4 moles
Q27: A 5.0-liter gas tank holds 1.7 moles
Q29: The root-mean-square speed (thermal speed)of a certain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents