Multiple Choice
3.00 moles of an ideal gas at a pressure of 250 kPa are held in a container of volume of 25.0 L.The ideal gas constant is R = 8.314 J/mol • K = 0.0821 L ∙ atm/mol • K,and 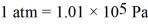 .The temperature of this gas is closest to
.The temperature of this gas is closest to
A) 240°C.
B) -180°C.
C) 480°C.
D) -1.0°C.
E) -22°C.
Correct Answer:
Verified
Related Questions
Q27: A 5.0-liter gas tank holds 1.7 moles
Q28: Sometimes an experiment requires a certain pure
Q29: The root-mean-square speed (thermal speed)of a certain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents