What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is 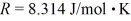 =
= 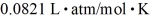 .
.
A) 1.40 kg/m3
B) 1.00 kg/m3
C) 1.20 kg/m3
D) 1.60 kg/m3
E) 1.80 kg/m3
Correct Answer:
Verified
Q33: An ideal gas is at a pressure
Q34: A sealed container holds 0.020 moles of
Q35: How many moles of water (H2O)molecules are
Q36: A hot air balloon has a volume
Q37: The figure shows a 50-kg frictionless cylindrical
Q39: The interior of a refrigerator has a
Q40: A bag of potato chips contains 2.00
Q41: On a hot summer day,the temperature is
Q42: Eleven molecules have speeds 16,17,18,...,26 m/s.Calculate the
Q43: What is the mean free path for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents