An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 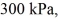 the initial volume is
the initial volume is 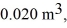 and the initial temperature is
and the initial temperature is 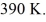 The final pressure is
The final pressure is 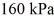 and the final temperature is
and the final temperature is 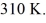 The change in the internal (thermal) energy of the gas is closest to
The change in the internal (thermal) energy of the gas is closest to
A) -3100 J.
B) -1800 J.
C) 3100 J.
D) 1800 J.
E) 0.00 J.
Correct Answer:
Verified
Q23: An ideal monatomic gas cools from 455.0
Q24: During an isothermal process,5.0 J of heat
Q25: A system has a heat source supplying
Q26: An ideal gas with γ = 1.67
Q27: In an isochoric process,the internal (thermal)energy of
Q29: A compression,at a constant pressure of 190
Q30: A quantity of ideal gas requires 800
Q31: A monatomic ideal gas undergoes an isothermal
Q32: The temperature of an ideal gas in
Q33: A certain amount of ideal monatomic gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents