An ideal Carnot engine operates between reservoirs having temperatures of 125°C and
-20°C.Each cycle the heat expelled by this engine is used to melt 30.0 g of ice at 0.00°C.The heat of fusion of water is 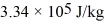 and the heat of vaporization of water is
and the heat of vaporization of water is 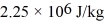 .
.
(a)How much work does this engine do each cycle?
(b)How much heat per cycle does this engine absorb at the hot reservoir?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q20: A real (non-Carnot)heat engine,operating between heat reservoirs
Q21: A Carnot air conditioner operates between an
Q22: A Carnot engine operating between a reservoir
Q23: A certain Carnot heat pump transfers energy
Q24: A Carnot engine operates between a high
Q26: What is the maximum theoretical efficiency possible
Q27: A Carnot refrigerator takes heat from water
Q28: A Carnot engine operates between reservoirs at
Q29: A perfect Carnot engine operates between the
Q30: A coal-fired plant generates 600 MW of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents