In an electroplating process,copper (ionic charge +2e,atomic weight 63.6 g/mol) is deposited using a current of 10.0 A.What mass of copper is deposited in 10.0 minutes? Avogadro's number is 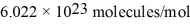 and e = 1.60 × 10-19 C.
and e = 1.60 × 10-19 C.
A) 3.96 g
B) 2.52 g
C) 0.99 g
D) 1.52 g
E) 1.98 g
Correct Answer:
Verified
Q4: The figure shows a steady electric current
Q5: A wire of resistivity ρ must be
Q6: If the current density in a wire
Q7: The figure shows two connected wires that
Q8: As current flows through a uniform wire,the
Q10: A narrow copper wire of length L
Q11: Two cables of the same length are
Q12: When a potential difference of 10 V
Q13: If a current of 2.4 A is
Q14: You are given a copper bar of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents