An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? ( h = 1.055 × 10-34 J • s, 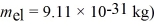
A) 5.26 × 105 m/s
B) 7.50 × 105 m/s
C) 5.26 × 107 m/s
D) 7.50 × 107 m/s
E) 5.26 × 109 m/s
Correct Answer:
Verified
Q28: A 440-nm spectral line is produced by
Q29: A beam of X-rays at a certain
Q30: A measurement of an electron's speed is
Q31: A nonrelativistic proton is confined to a
Q32: X-rays of energy 2.9 × 104 eV
Q33: A certain particle's energy is measured by
Q35: A photon of wavelength 18.0 pm is
Q36: The excited state of a certain atom
Q37: The energy of an electron state has
Q38: A photon of wavelength 29 pm is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents