A hydrogen atom is in its n = 2 excited state when its electron absorbs a photon of energy 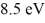 .What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
.What is the energy of the resulting free electron? The lowest level energy state of hydrogen is -13.6 eV.(h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J)
A) 5.1 eV
B) 6.6 eV
C) 6.9 eV
D) 7.6 eV
Correct Answer:
Verified
Q1: A hydrogen atom is excited to the
Q3: At absolute temperature T,a black body radiates
Q4: A nonrelativistic electron and a nonrelativistic proton
Q4: If the accuracy in measuring the position
Q6: The Bohr radius of the hydrogen atom
Q7: Light shines through atomic hydrogen gas.It is
Q8: In the vicinity of what frequency does
Q10: The energy of the ground state in
Q11: A perfectly black body at 100°C emits
Q17: If the accuracy in measuring the velocity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents