The excited state of a certain atom is 3.2 eV ± 0.21 eV.( h = 1.055 × 10-34 J • s = 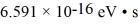 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
(a)What is the average lifetime of this state?
(b)If the excited energy were doubled to 6.4 eV ± 0.21 eV,how would the lifetime be affected?
Correct Answer:
Verified
(b)unchan...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q33: A measurement of an electron's speed is
Q34: A nonrelativistic electron is accelerated from rest
Q35: How fast must a nonrelativistic electron move
Q36: What is the energy of a photon
Q37: In a double slit experiment,a beam of
Q39: Electrons emerge from an electron gun with
Q40: A nonrelativistic electron has a kinetic energy
Q41: A collection of atoms has 20% of
Q42: The wavelength of a ruby laser is
Q43: In a ruby laser,an electron jumps from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents