The normalized wave function for a hydrogen atom in the 1s state is given by 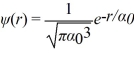 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
A) 2.3 × 10-5
B) 1.2 × 10-5
C) 1.7 × 10-5
D) 4.6 × 10-5
E) 3.5 × 10-5
Correct Answer:
Verified
Q29: The only INVALID electron state and shell
Q30: What is the minimum speed needed by
Q31: What is the greatest total angular momentum
Q32: How many electrons can be found with
Q33: The magnitude of the orbital angular momentum
Q35: How many electrons does it take to
Q36: How many possible sets of quantum numbers
Q37: An atom with 5 electrons is in
Q38: The magnitude of the orbital angular momentum
Q39: The correct ground state electron configuration of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents