The neutral deuterium atom, 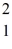 H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the
H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the 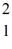 H nucleus?
H nucleus? 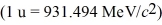
A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV
Correct Answer:
Verified
Q22: The carbon in your body was formed
Q23: A certain nucleus containing 8 protons and
Q24: What would be the expected radius of
Q25: The following masses are known: 
Q26: How much energy is released when 1.40
Q28: Which of the following descriptions best describes
Q29: Two identical nuclei of mass 18 u
Q30: Plutonium-239 decays into uranium-235 plus an alpha
Q31: Uranium-238 decays into thorium-234 plus an alpha
Q32: How does the mass of the products
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents