The stability of 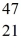 Sc with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Sc with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 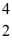 He: 4.002603 u
He: 4.002603 u 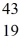 K: 42.960717 u
K: 42.960717 u 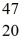 Ca: 46.954543 u
Ca: 46.954543 u 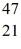 Sc 46.952409 u
Sc 46.952409 u 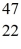 Ti: 46.951764 u The
Ti: 46.951764 u The 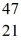 Sc nuclide is
Sc nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+ decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer:
Verified
Q49: How many days are required for a
Q50: A radioactive atom has 98 protons and
Q51: A sphere made of a radioactive isotope
Q52: Fermium-253 has a half-life of 3.00 d.A
Q53: Rutherfordium-261 has a half-life of 1.08 min.How
Q55: In a laboratory accident a work area
Q56: The radioactivity due to carbon-14 measured in
Q57: The material used in certain nuclear bombs
Q58: A certain substance has a half-life of
Q59: An isotope of Tc having a half-life
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents