Short Answer
In the nuclear reaction
n + 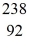 U → X + 2e-
U → X + 2e-
n is a neutron and e- is an electron,and the neutrinos have not been shown.Determine the atomic mass and atomic number of the missing nuclear product X,and write X in the standard form.It is NOT necessary to identify which atom X is.
Correct Answer:
Verified
Related Questions
Q68: An ancient rock is found to contain
Q69: Living matter has 1.3 × 10-10 %
Q70: When a neutron (n)collides with a uranium-235
Q71: The reaction energy (Q value)for a particular
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents