If we double the root-mean-square speed (thermal speed) of the molecules of a gas,then
A) its temperature must increase by a factor of 4.
B) its temperature must increase by a factor of 2.
C) its temperature must increase by a factor of 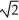 .
.
D) its pressure must increase by a factor of 2.
E) its pressure must increase by a factor of 4.
Correct Answer:
Verified
Q5: A sealed 26- m3 tank is filled
Q7: If the temperature of a fixed amount
Q9: A weather balloon contains 12.0 m3 of
Q13: A container is filled with a mixture
Q20: A sample of an ideal gas is
Q24: An ideal gas is at a pressure
Q36: Sometimes an experiment requires a certain pure
Q41: Suppose that the build-up of fatty tissue
Q53: Motor oil,with a viscosity of 0.250 N
Q791: A vertical tube that is closed at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents