Classify each of the following as ground state, excited state, or impossible electronic configurations.
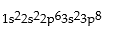
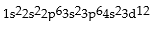
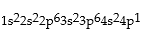
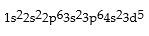
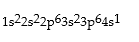
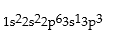
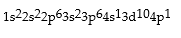
Correct Answer:
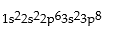
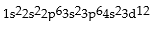
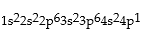
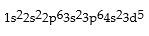
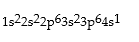
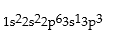
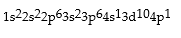
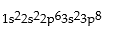
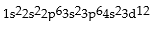
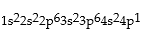
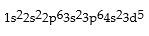
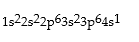
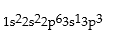
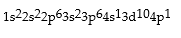
Q42: The wavelengths in infrared radiation are larger
Q53: Both the energy and the wavelength of
Q64: The number of electrons in the valence
Q66: Should an oxygen atom (O) gain two
Q82: Shells get larger as the principal quantum
Q83: The red light is the element that
Q84: Write the complete electronic ground state configuration
Q86: Match every binary compound in the first
Q87: List the sublevels of the n =
Q94: Modern scientists accept the notion that electrons
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents