A 2.00 g sample of a non-electrolyte (molar mass = 118) is dissolved in 55.0 g benzene (Kf of benzene is 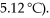 What is the freezing point depression of the solution?
What is the freezing point depression of the solution?
A) 1.58 °C
B) 3.16 °C
C) 0.79 °C
D) 2.33 °C
Correct Answer:
Verified
Q43: Transparent solutions are examples of homogeneous matter.
Q45: Vinegar is a liquid solution where acetic
Q51: 10.0 mL 0.5 M sulfuric acid neutralizes
Q55: 10.0 mL 0.5 M HCl neutralizes _
Q56: 10.0 mL 0.5 M sulfuric acid neutralizes
Q71: Nonpolar solutes will usually dissolve in polar
Q74: A mixture is not necessarily a solution.
Q76: Polar solutes will usually dissolve in polar
Q79: Ca2+ is expected to have a greater
Q97: There is no difference between the "molarity"
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents