The compound HA is an acid that is soluble in water. Which of the "beakers" below shows HA behaving as a strong acid in water?
A) 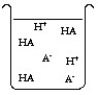
B) 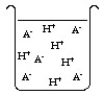
C) 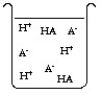
D) 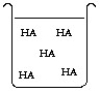
Correct Answer:
Verified
Q12: Which of the following is a non-electrolyte
Q13: Which of the following could be considered
Q14: Which of the following is not a
Q15: What is the hydroxide-ion concentration of an
Q16: Which of the following is not a
Q18: Which of the following conjugate pairs would
Q19: Which of the following is a diprotic
Q20: Which of the following produces more than
Q22: Which of the following qualifies as a
Q44: The pH of natural rainwater is close
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents