Consider the following van der Waals coefficients: 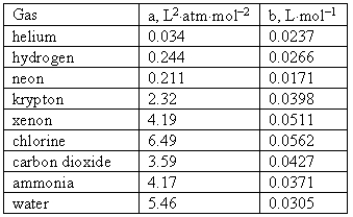 Which of the following gases has the largest attractive forces?
Which of the following gases has the largest attractive forces?
A) Neon
B) Ammonia
C) Chlorine
D) Water
E) Helium
Correct Answer:
Verified
Q62: Which of the following gases would not
Q63: How many liters of carbon dioxide
Q65: Helium,H2,and neon all have very small values
Q66: What is the molar volume of a
Q69: How many liters of nitrogen gas
Q73: A 1.00-L sample of C2H4(g)at 2.00 atm
Q73: A plot of the Maxwell distribution for
Q77: Which molecules of the following gases will
Q79: Consider two flasks at 25
Q80: Which of the following gases would you
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents