The phase diagram for CO2 is given below.The triple point is at 5.1 atm and 217 K. 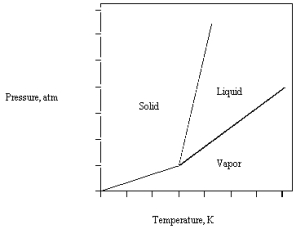 What happens if CO2(l) at 30 atm and 450 K is released into a room at 1 atm and 298 K?
What happens if CO2(l) at 30 atm and 450 K is released into a room at 1 atm and 298 K?
A) The liquid vaporizes.
B) The liquid remains stable.
C) The liquid and vapor are in equilibrium.
D) The liquid and solid are in equilibrium.
E) The liquid freezes.
Correct Answer:
Verified
Q23: For a one-component system, at the triple
Q32: Consider the phase diagram for sulfur in
Q35: The phase diagram for sulfur is given
Q36: The phase diagram for carbon dioxide
Q37: the triple point for water,4.6 Torr
Q44: Aqueous ammonia (28%)is 15.0 M and has
Q44: The enthalpy of hydration of AgBr
Q47: Benzene would likely dissolve which of the
Q49: For CaCl2,the enthalpies of hydration and
Q57: Calculate the molality of perchloric acid in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents