The following phase diagram is for a pure substance. 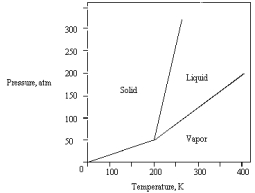 Below what temperature does the substance exist as a liquid?
Below what temperature does the substance exist as a liquid?
A) 150 K
B) 200 K
C) 300 K
D) 350 K
E) 400 K
Correct Answer:
Verified
Q65: Which of the following pairs have a
Q79: Consider the diagram below. Q81: Because a solute increases the entropy of Q83: The solubility of oxygen at a certain Q85: On what conditions does the location Q87: The increase in entropy of the system Q87: Which of the following has the smallest Q88: Using a mass of 500.0 g of Q91: The lattice enthalpy and the enthalpy of Q92: With which of the following solutes can![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents