The fractional composition diagram for the amino acid alanine is given below. 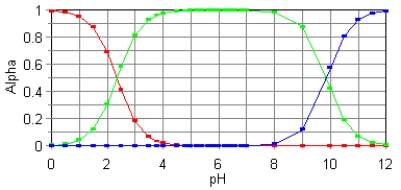 Write the structure of the dominant species at pH 1,6,and 12,respectively.
Write the structure of the dominant species at pH 1,6,and 12,respectively.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q62: The pH of 0.010 M H3PO4(aq)is 2.24;
Q64: If
Q65: The amino acid alanine,HOOC-CH(CH3)NH3+,has Ka1 =
Q67: Calculate the equilibrium constant for the
Q67: The pH of 0.010 M H3PO4(aq)is 2.24.Estimate
Q68: Estimate the pH of 0.10 M Na2HPO4(aq)given
Q69: The amino acid alanine,HOOC-CH(CH3)NH3+,has Ka1 =
Q70: In a solution labeled "0.0018 M barium
Q71: All of the following are Lewis bases
Q71: the pH of 0.10 M and 0.40
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents