The following compounds are available as 0.10 M aqueous solutions. 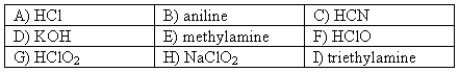 Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Pick two solutions that could be used to prepare a buffer with a pH of ~ 4.
Correct Answer:
Verified
Q29: The following compounds are available as 0.10
Q30: For NH3,pKb = 4.74.What is the pH
Q35: If a small amount of a strong
Q36: A buffer contains equal concentrations of a
Q37: If a small amount of a strong
Q38: Calculate the ratio of the molarities of
Q39: For HF,pKa = 3.45.What is the pH
Q120: Which of the following mixtures gives a
Q131: Which of the following mixtures gives a
Q139: A buffer solution contains 0.75 mol KH2PO4
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents