The titration curve for the titration of 0.100 M H2SO3(aq) with 0.100 M KOH(aq) is given below. 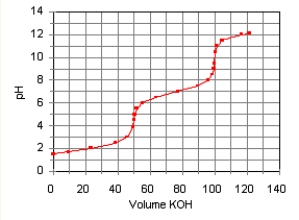
The major species in solution after 75 mL of KOH(aq) has been added are
A) HSO3-(aq) and Na+(aq) .
B) SO32-(aq) ,and Na+(aq) .
C) SO32-(aq) ,OH-(aq) ,and Na+(aq) .
D) H2SO3(aq) ,HSO3-,and Na+(aq) .
E) HSO3-(aq) ,SO32-(aq) ,and Na+(aq) .
Correct Answer:
Verified
Q56: The titration curve for the titration of
Q62: Calculate the value of the equilibrium constant
Q64: The titration curve for the titration of
Q65: Consider the titration of 15.0 mL of
Q125: Calculate the equilibrium constant for the reaction
Q144: Consider the titration of 50.0 mL of
Q153: Consider the titration of 10.0 mL of
Q156: A certain weak acid has a Ka
Q159: Consider the titration of 50.0 mL of
Q178: Calculate the solubility product of calcium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents