The titration curve for the titration of 0.100 M Na2CO3(aq) with 0.100 M HClO4(aq) is: 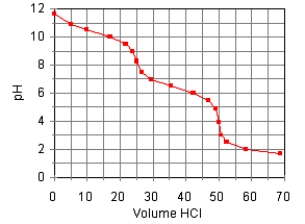 Estimate pKb2.
Estimate pKb2.
A) 7.6
B) 10.3
C) 6.4
D) 8.5
E) 3.7
Correct Answer:
Verified
Q67: You have available the following reagents as
Q68: The titration curve for the titration of
Q69: Silver bromide is most soluble in
A)pure H2O(l).
B)dilute
Q70: If equal volumes of 0.004 M Pb(NO3)2(aq)and
Q71: The Cu2+ ion can be separated from
Q74: If you wish to increase the solubility
Q75: What is the relationship between the solubility
Q158: What is the equilibrium constant for the
Q170: If the molar solubility of the compound
Q180: Which of the following water-insoluble salts is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents