The concentration-time dependence for two first order reactions is: 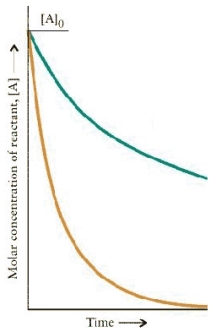
Which reaction has the greater t½?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: If the rate of a reaction increases
Q4: Given: 2NO2(g)+ F2(g)
Q5: The concentration-time dependence for a first-order reaction
Q7: For the reaction S2O82-(aq)+ 3I-(aq)
Q8: The reaction 2NO(g)+ 2H2(g)
Q9: Given:
2O3(g)
Q12: The concentration-time curves for two sets of
Q13: For the reaction
2NO(g)+ 2H2(g)
Q14: The rate of formation of oxygen
Q15: Given:
4Fe2+(aq)+ O2(aq)+ 2H2O(l)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents