Magnesium burns in air with a dazzling brilliance to produce magnesium oxide: 2 Mg(s) + 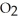 (g) → 2 MgO(s)
(g) → 2 MgO(s)
When 3.50 g of magnesium burns,the theoretical yield of magnesium oxide is ________ g.
A) 3.50
B) 5.80
C) 0.144
D) 2.90
E) 11.6
Correct Answer:
Verified
Q33: How many moles of BCl3 are needed
Q79: Consider the following reaction.How many moles of
Q81: Balance the chemical equation given below,and determine
Q82: Lithium and nitrogen react in a combination
Q83: When silver nitrate reacts with barium chloride,silver
Q86: Which substance is the limiting reactant when
Q87: If 2352 grams of FeS2 is allowed
Q88: Calcium oxide reacts with water in a
Q89: Give the theoretical yield,in moles,of CO2 from
Q122: Dinitrogen monoxide gas decomposes to form nitrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents