A fixed amount of gas at 25.0°C occupies a volume of 10.0 L when the pressure is 751 torr.Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 9.25 L at a constant temperature of 25.0°C.
A) 812 torr
B) 0.123 torr
C) 6.95 × 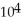 torr
torr
D) 695 torr
E) 1.07 torr
Correct Answer:
Verified
Q56: Determine the volume of SO2 (at STP)formed
Q57: Convert 2.00 atm to psi.
A) 59.8 psi
B)
Q58: Which of the gases in the graph
Q59: The atmospheric pressure is 715 mm Hg.What
Q60: Convert 2.00 atm to mm Hg.
A) 760
Q62: A syringe contains 0.65 moles of He
Q63: A scuba diver experiences _ as she
Q64: Calculate the temperature,in K,of 2.20 moles of
Q66: A fixed amount of gas at 25.0°C
Q109: A basketball is inflated to a pressure
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents