A sample of air from a home is found to contain 4.3 × 10-6 of carbon monoxide.This means that if the total pressure is 735 torr,then the partial pressure of CO is ________ torr.
A) 3.2 × 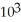
B) 3.2 × 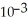
C) 3.2
D) 5.9 × 103
E) 1.7 × 108
Correct Answer:
Verified
Q49: In the laboratory,hydrogen gas is usually made
Q125: Zinc reacts with aqueous sulfuric acid to
Q126: The mole fraction of oxygen in dry
Q127: How many grams of zinc metal are
Q128: The mole fraction of argon in dry
Q129: What is the total pressure in a
Q131: A 1.000 kg sample of nitroglycerine,C3H5N3O9,explodes and
Q132: The concentration of water vapor in a
Q133: Zinc reacts with aqueous sulfuric acid to
Q134: A balloon contains 0.76 mol O2,0.18 mol
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents