Multiple Choice
Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn(s) + 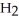
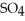 (aq) → Zn
(aq) → Zn 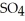 (aq) +
(aq) + 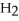 (g)
(g)
In an experiment,201 mL of wet 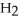 is collected over water at 27°C and a barometric pressure of
is collected over water at 27°C and a barometric pressure of 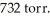 How many grams of Zn have been consumed? The vapor pressure of water at 27°C is
How many grams of Zn have been consumed? The vapor pressure of water at 27°C is 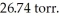
A) 4.18 × 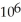 g
g
B) 0.496 g
C) 496 g
D) 377 g
E) 3.77 × 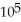 g
g
Correct Answer:
Verified
Related Questions
Q55: When 15.0 g of zinc metal reacts
Q120: The density of chlorine gas at 0.866
Q121: The concentration of ozone in a sample
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents