What volume of benzene (C6H6,d= 0.88 g/mL,molar mass = 78.11 g/mol) is required to produce 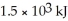 of heat according to the following reaction? 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(g) ΔH°rxn = -6278 kJ
of heat according to the following reaction? 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(g) ΔH°rxn = -6278 kJ
A) 75 mL
B) 37 mL
C) 21 mL
D) 19 mL
E) 42 mL
Correct Answer:
Verified
Q28: Using the following equation for the combustion
Q29: Using the following equation for the combustion
Q30: A 35.6 g sample of ethanol (C2H5OH)is
Q31: A 100.0 mL sample of 0.300 M
Q32: Given w = 0,an endothermic reaction has
Q34: Which of the following statements is TRUE?
A)
Q35: Two aqueous solutions are both at room
Q36: According to the following thermochemical equation,what mass
Q37: According to the following reaction,how much energy
Q38: Which statement is FALSE?
A) An exothermic reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents