A 4.50-g sample of copper metal at 25.0°C is heated by the addition of 145 J of energy.The final temperature of the copper is ________°C.The specific heat capacity of copper is 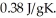
A) 37.2
B) 25.0
C) 59.8
D) 110
E) 84.8
Correct Answer:
Verified
Q85: How much energy is evolved during the
Q86: Determine the specific heat capacity of an
Q87: Calculate the change in internal energy (ΔE)for
Q88: Which of the following processes is endothermic?
A)
Q89: The combustion of titanium with oxygen produces
Q91: What is the enthalpy change (in kJ)of
Q92: A sample of copper absorbs 43.6 kJ
Q93: When 1.365 g of anthracene,C14H10,is combusted in
Q94: It takes 11.2 kJ of energy to
Q95: The specific heat capacity of methane gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents