When 5.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00°C to 61.16°C.If the specific heat of the solution is 4.18 J/(g ∙ °C) ,calculate 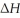 for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
for the reaction,as written. Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g) 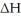 = ?
= ?
A) -431 kJ
B) -3.14 kJ
C) +3.14 kJ
D) +431 kJ
Correct Answer:
Verified
Q17: When 10.0 mol of benzene is vaporized
Q98: Which of the following processes is endothermic?
A)
Q99: Using the following equation for the combustion
Q100: A sample of copper absorbs 43.6 kJ
Q101: How much energy is required to decompose
Q102: Using the following thermochemical equation,determine the amount
Q104: According to the following thermochemical equation,what mass
Q105: 8.000 moles of H2(g)react with 4.000 mol
Q107: How much energy is evolved during the
Q108: When 0.500 mol of CH4(g)reacts with excess
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents