Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 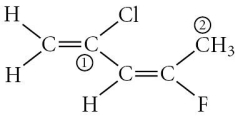
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2= bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw
Correct Answer:
Verified
Q12: Place the following in order of increasing
Q13: How many of the following molecules are
Q14: A molecule with a seesaw molecular geometry
Q15: Consider the molecule below.Determine the molecular geometry
Q16: Place the following in order of increasing
Q18: Give the molecular geometry and number of
Q19: Place the following in order of increasing
Q20: How many of the following molecules are
Q21: How many of the following molecules have
Q22: Give the hybridization for the S in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents