Use the molecular orbital diagram shown to determine which of the following is most stable. 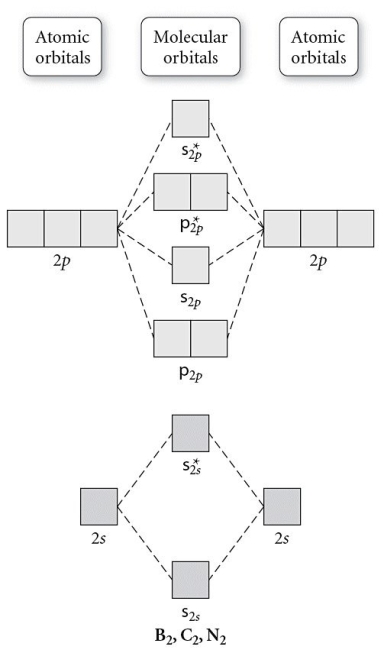
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
Correct Answer:
Verified
Q41: Draw the molecular orbital diagram shown to
Q42: Give the number of sigma bonds and
Q43: Draw the molecular orbital diagram shown to
Q44: Determine the electron geometry (eg)and molecular geometry
Q45: Give the approximate bond angle for a
Q47: Give the approximate bond angle for a
Q48: Determine the electron geometry (eg)and molecular geometry
Q49: Determine the electron geometry (eg)and molecular geometry
Q50: Determine the electron geometry (eg)and molecular geometry
Q51: Draw the molecular orbital diagram shown to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents