Ethanol ( 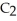
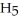 OH) melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/gK and 2.3 J/gK,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -125°C to liquid ethanol at -80°C?
OH) melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/gK and 2.3 J/gK,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -125°C to liquid ethanol at -80°C?
A) 207.3 kJ
B) -14.2 kJ
C) 4.95 kJ
D) 2224 kJ
E) 7.24 kJ
Correct Answer:
Verified
Q109: The freezing point of water is _.
A)
Q110: The enthalpy change for converting 1.00 mol
Q111: Which substance below has the strongest intermolecular
Q112: Define triple point.
A) the temperature, pressure, and
Q113: Calculate the total quantity of heat required
Q115: Identify the compound with the highest boiling
Q116: The fluorocarbon C2Cl3F3 has a normal boiling
Q117: The melting point of water is _.
A)
Q118: The normal boiling point of water is
Q119: Consider the phase diagram below.If the dashed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents