Consider the phase diagram below.If the dashed line at 1 atm of pressure is followed from 80 to 350°C,what phase changes will occur (in order of increasing temperature) ? 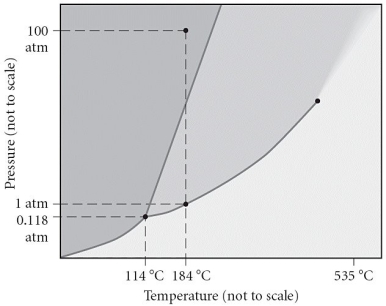
A) condensation, followed by sublimation
B) sublimation, followed by fusion
C) vaporization, followed by deposition
D) fusion, followed by vaporization
E) No phase change will occur under the conditions specified.
Correct Answer:
Verified
Q114: Ethanol ( Q115: Identify the compound with the highest boiling Q116: The fluorocarbon C2Cl3F3 has a normal boiling Q117: The melting point of water is _. Q118: The normal boiling point of water is Q120: How much heat is released when 115 Q121: Match the following. Q122: Match the following. Q123: Define boiling point of a liquid. Q124: Define volatile.![]()
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents