Which of the following represents the equation for a zero-order half-life?
A) t 1/2 = 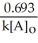
B) t 1/2 = 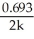
C) t 1/2 = 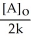
D) t 1/2 = 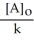
E) t 1/2 = 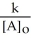
Correct Answer:
Verified
Q88: The combustion of ethylene proceeds by the
Q96: The isomerization of methylisonitrile to acetonitrile CH3NC
Q113: How many half-lives are required for the
Q115: The decomposition of Q116: SO2Cl2 decomposes in the gas phase by Q117: The rate constant for a first-order reaction Q120: The rate constant for a zero-order reaction Q121: Derive an expression for a "1/4-life" for Q122: Which rate law is bimolecular? Q123: Hydrogen iodide decomposes at 800 K via![]()
A) rate =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents