Express the equilibrium constant for the following reaction. 14 P(g) + 21 Cl2(g) ⇌ 14 PCl3(g)
A) K = 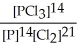
B) K = 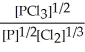
C) K = 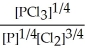
D) K = 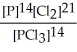
E) K = 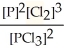
Correct Answer:
Verified
Q72: Consider the following reaction at equilibrium.What effect
Q73: Consider the following reaction at equilibrium.What effect
Q74: Express the equilibrium constant for the following
Q75: Consider the following reaction at equilibrium.What effect
Q76: Express the reverse equilibrium constant for the
Q78: Express the reverse equilibrium constant for the
Q79: Express the equilibrium constant for the following
Q80: The equilibrium constant is given for one
Q81: What is Δn for the following equation
Q82: The decomposition of ammonia is: 2 NH3(g)⇌
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents