At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345. 2 SO2(g) + O2(g) ⇌ 2 SO3(g)
At equilibrium,the partial pressure of SO2 is 35.0 atm and that of 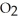 is 15.9 atm.The partial pressure of SO3 is ________ atm.
is 15.9 atm.The partial pressure of SO3 is ________ atm.
A) 82.0
B) 4.21 × 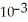
C) 192
D) 6.20 × 10-4
E) 40.2
Correct Answer:
Verified
Q88: What is Δn for the following equation
Q89: What is △n for the following equation
Q90: What is Δn for the following equation
Q91: For the reaction: N2(g)+ 2 O2(g)⇌ 2
Q92: What is Δn for the following equation
Q94: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q95: What is Δn for the following equation
Q96: What is Δn for the following equation
Q97: What is △n for the following equation
Q98: What is Δn for the following equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents