The pH of an aqueous solution at 25.0°C is 10.66.What is the molarity of 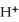 in this solution?
in this solution?
A) 2.2 × 10-11
B) 4.6 × 10-4
C) 3.3
D) 1.1 × 10-13
E) 4.6 × 1010
Correct Answer:
Verified
Q57: What is the pH of a 0.020
Q105: Calculate the pH of a 1.60 M
Q106: What is the hydroxide ion concentration and
Q107: What are the hydronium ion concentration and
Q108: Determine the pH of a 0.741 M
Q110: Calculate the molarity of hydroxide ion in
Q112: What is the hydronium ion concentration of
Q113: The acid-dissociation constant at 25.0°C for hypochlorous
Q115: Determine the [OH⁻] concentration in a 0.235
Q180: What is the hydronium ion concentration of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents