What is the pH of a solution made by mixing 30.00 mL of 0.10 M acetic acid with 50.00 mL of 0.100 M KOH? Assume that the volumes of the solutions are additive.Ka = 1.8 × 10-5 for 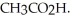
A) 8.26
B) 9.26
C) 11.13
D) 12.40
Correct Answer:
Verified
Q81: A 25.0 mL sample of 0.150 M
Q117: A 100.0 mL sample of 0.10 M
Q118: A 1.0 L buffer solution is 0.060
Q119: A 1.0 L buffer solution is 0.250
Q120: A 900.0 mL sample of 0.18 M
Q121: A 25.0-mL sample of 0.150 M butanoic
Q123: A 100.0 mL sample of 0.20 M
Q124: What is the pH of the resulting
Q125: A 600.0 mL sample of 0.20 M
Q127: A 200.0 mL sample of 0.20 M
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents