Write a nuclear equation to describe the spontaneous fission of 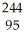 Am to form I-134 and Mo-107.Determine how many neutrons are produced in the reaction.
Am to form I-134 and Mo-107.Determine how many neutrons are produced in the reaction.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:
Verified
Q28: Calculate the mass defect in Fe-56 if
Q29: Identify the technique used to predict the
Q30: Define mass defect.
A) the difference in mass
Q31: The splitting of the uranium atom is
Q32: Write a nuclear equation to describe the
Q34: A geological sample is found to have
Q35: Fluorine-18 undergoes positron emission with a half-life
Q36: The splitting of a heavy nucleus to
Q37: Calculate the mass defect in Mo-96 if
Q38: Identify the nuclide that has the shortest
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents