Write a balanced equation for the calcination of Al(OH) 3.
A) 2 Al(OH) 3(s) 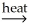 Al2O3(s) + 3 H2O(g)
Al2O3(s) + 3 H2O(g)
B) Al(OH) 3(s) + SiO2(s) 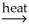 AlSiO3(s) + 3 H2O(g)
AlSiO3(s) + 3 H2O(g)
C) 2 Al(OH) 3(s) + O2(g) 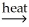 Al2O3(s) + 3 H2O(g)
Al2O3(s) + 3 H2O(g)
D) Al(OH) 3(s) + C(s) 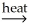 Al2O3(s) + CO2(g) + H2O(g)
Al2O3(s) + CO2(g) + H2O(g)
E) None of the above represents calcination.
Correct Answer:
Verified
Q3: Metals make up only _ percent of
Q4: Identify the substances which are homogeneous,naturally occurring,crystalline
Q5: Write a balanced reaction for the smelting
Q6: Identify the element that has the most
Q7: Which of the following is TRUE concerning
Q9: Identify the substances which contain a high
Q10: Which of the following describes slag?
A) a
Q11: Which of the following is TRUE of
Q12: In an interstitial alloy,
A) one nonmetal substitutes
Q13: Which of the following is TRUE concerning
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents