What is the atomic symbol for an element with 16 protons and 17 neutrons?
A) 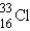
B) 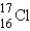
C) 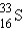
D) 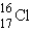
E) 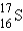
Correct Answer:
Verified
Q8: Which of the following atoms contains the
Q8: How many protons are there in an
Q9: Two isotopes of a given element will
Q10: Atoms consist of three fundamental particles.What are
Q11: An atom that has the same number
Q13: A neutral atom of the isotope 197Au
Q14: If two different isotopes have the same
Q15: Rank the subatomic particles from least to
Q16: The atomic number of fluorine is _.
A)
Q17: Atomic number is the_ in the nucleus
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents