Write a balanced equation for the reaction that occurs when hydrogen sulfate ion behaves as a Brønsted-Lowry acid in water.
A) HSO4-(aq) + H2O( 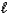 )
) 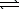 H2SO4(aq) + H3O+(aq)
H2SO4(aq) + H3O+(aq)
B) SO42-(aq) + H2O( 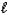 )
) 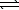 HSO4-(aq) + OH-(aq)
HSO4-(aq) + OH-(aq)
C) HSO4-(aq) + H2O( 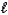 )
) 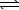 SO42-(aq) + H3O+(aq)
SO42-(aq) + H3O+(aq)
D) HSO4-(aq) + H2O( 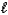 )
) 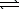 H2SO4(aq) + OH-(aq)
H2SO4(aq) + OH-(aq)
E) H2SO4(aq) + H2O( 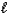 )
) 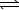 HSO4-(aq) + H3O+(aq)
HSO4-(aq) + H3O+(aq)
Correct Answer:
Verified
Q31: Nitric acid is the product of the
Q49: Which of the following would
Q50: What is the net ionic equation
Q51: Which of the following is a
Q52: When solutions of barium chloride and
Q53: Which equation best represents the
Q55: What are the spectator ions in the
Q56: Metal oxides react with water to produce
Q57: What base results when Li2O reacts with
Q77: In the reaction of acetic acid with
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents